🚧 What Is Quarantine in Food Manufacturing?
Quarantining refers to the temporary isolation of food products, ingredients, or packaging materials that may pose a risk to safety, quality, or regulatory compliance. These items are held back from distribution or further processing until they are either cleared or disposed of.
This process is not about punishment—it’s about prevention.

🔍 Why Quarantine Matters
Quarantine protocols serve several vital functions:
- Ensuring Regulatory Compliance: Items that don’t meet specifications or labeling requirements can be held until corrected.
- Preventing Contamination Spread: If a batch is suspected of contamination (e.g., microbial, chemical, or physical), quarantining prevents it from entering the supply chain.
- Supporting Traceability: Quarantined items should be tagged and logged, aiding root cause analysis and recall management.
- Protecting Brand Reputation: Catching issues before products reach consumers helps avoid costly recalls and public backlash.
🧰 Common Triggers for Quarantine
Products may be quarantined due to:
- Awaiting / Failed quality control tests (e.g., pH, moisture, microbial load)
- Packaging defects or labeling errors
- Suspicion of allergen cross-contact
- Deviation from critical control points (CCPs) in HACCP plans
- Supplier non-compliance or documentation gaps
🗂️ How Quarantine Is Managed
Effective quarantine systems rely on structured protocols and traceability:
- Physical Segregation: Items are stored in clearly marked, restricted-access areas.
- Clear Labeling: “Hold,” “Quarantine,” or “Do Not Use” tags / tape are applied visibly to affected items.
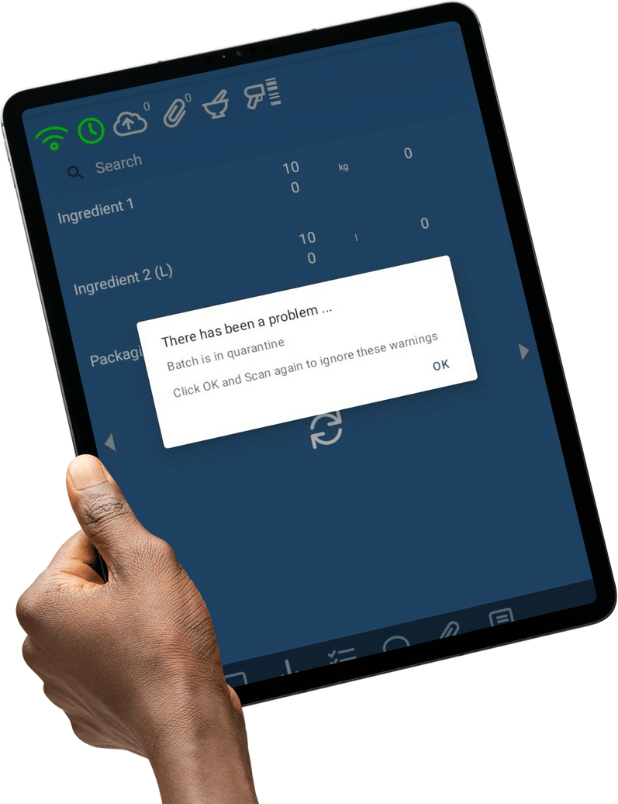
- Digital Tracking: ERP systems, like NotaZone, allow you to digitally move the item to a Quarantine location. (If the item is scanned to a order whilst in Quarantine, this will result in a pop up warning to alert the user the item should not be used).
- Release Procedures: Items are only released from the Quarantine location after testing or corrective actions.
- Rejections: Where a product fails release procedures, ensure follow up actions like disposal or rework are clearly documented.
- Corrective Action Logging: notes and documents relating to the quarantine investigation can be uploaded to the batch as evidence.
🧠 Best Practices for Implementation
To ensure quarantine protocols are robust and efficient:
- Train staff on identification and escalation procedures.
- Have clearly labelled Quarantine locations, in both your physical facility and on your NotaZone system.
- Conduct regular audits of quarantine areas (comparing physical stock to the what is recorded on NotaZone as in the quarantine location).
- Define clear criteria for release, rework, or disposal.
- Maintain records within NotaZone to show when criteria met, and item released from Quarantine (or reworked / disposed of).
🥽 Quarantine as a Culture of Safety
Quarantining isn’t just rolling out the safety net after the fact— it’s a reflection of a proactive safety culture. When staff are given the power to spot trouble and pop potential hazards into isolation, utilising tools like NotaZone, the whole operation becomes more resilient.
So in the unpredictable world of food, where a single slip-up can cause chaos, ensure your Team don't have questions about quarantine.
